It’s a very human thing to discover an exciting new prospect and want to share it as soon as possible so that we can all reap the rewards. This happens a lot in science as well: when thalidomide was put  on the market decades ago, we wanted pregnant women to stop having morning sickness. Instead, the lack of research and long-term effects meant that birth defects were uncommonly high among those taking the drug. Had we taken a little more care with manufacturing or research, it may not have happened that way. The world got excited, and many newborn children paid the price of the immaturity of the compound’s data.
on the market decades ago, we wanted pregnant women to stop having morning sickness. Instead, the lack of research and long-term effects meant that birth defects were uncommonly high among those taking the drug. Had we taken a little more care with manufacturing or research, it may not have happened that way. The world got excited, and many newborn children paid the price of the immaturity of the compound’s data.
It’s an ethical question: when do we have “enough” research to peg a new compound as safe to use? If we find a new way to preserve food that seems reasonably safe, should we bother to test it long-term before putting it out on the market? This is the exact issue that occurred when butter substitutes like margarine and Crisco hit the market. Their properties are amazing to behold and the chemistry is an accomplishment of science. Yet, if only we had tested them a bit longer before putting them on the market…
What they were going for…
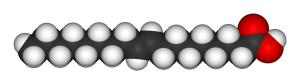
And what they ended up with. Note the weird bond in the middle — it’s a trans-fat!
Alas, the heart of this matter is what we call “trans fats,” which nearly everyone has heard of at some point. They were banned in some states, and the FDA requires you to list the amount of trans fats in your food products. What makes them so “dangerous?” In short, they’re a fabrication of fats, and unfortunately for us, they’re not an exact replica. The differences that trans fats bring in their molecular structure are responsible for the (justly so) backlash against them, and their eventual demonization. 40 years ago, scientistsand laypersons alike were in awe of the amazing substance called “partially hydrogenated oil.” It’s time to delve into what that really means, and how it makes trans fats dangerous.
Quiz: at room temperature, is olive oil a liquid or a solid? How about bacon grease? The difference is that bacon grease is called a “saturated” fat, meaning that the long carbon chain that makes up the fat is filled to its limit with hydrogen atoms. Each carbon in the chain can hold 2 hydrogens, as well as make 2 connections to other carbons. These chains end up long and very straight, so that when a bunch of them come up next to each other, they pack tightly enough to solidify — this is why animal fats are solid at room temperature. It’s simply because the fat molecules are better lego pieces with each other than other fats.
Those other fats, of course, are unsaturated fats. They’re mostly vegetable or nut oils, like olive, sesame, almond, peanut, and soy. They’re unsaturated because they’re not packing their carbons to the brim with hydrogens — and when a carbon doesn’t have enough hydrogens, it has to make a bond to a carbon it’s already attached to, which gives it a double bond with another carbon. To provide some background, carbon must make 4 bonds when it can, otherwise, it’s extremely reactive. It doesn’t care if it makes 4 separate bonds, or 2 double bonds — as long as it’s making 4 bonds in some way or another, it’s happy and stable.
We said these fats aren’t as good “lego” pieces as saturated fats are. Why’s that? Well, when carbons make a double bond to each other, their orientations change: instead of being perfectly straight, they tend to bend at angles and kink up here and there. They take up a lot more space because their shape has been forced to change. This means they won’t pack as tightly (five people standing in a phone booth with arms at their sides would pack in tightly, but two people with their arms out could not easily fit in this same space!), and so they don’t solidify. Olive oil is still liquid at room temperature because it can’t pack tightly enough.
By a miracle of science, we figured out how to shoot up unsaturated fats with more hydrogens molecules. In other words, we figured out how to transform olive oil into butter – it would be a solid at room temperature. It’s great for preserving food since it won’t get all liquidy on you, and it’s the reason that Crisco stays solid even when left on the counter. It’s why you never have to stir your peanut butter in the jar unless you buy a jar with only a single ingredient on the label. But we didn’t quite hit the mark when we employed this technology, and here’s where it gets a little scary.
It turns out we’re not perfect at shooting up those fats with hydrogens, and we miss the 100% mark some of the time, leaving those fats “partially hydrogenated,” which gives them a unique molecular structure and shape. When that happens, we’ve created a type of fat that the human body has never before encountered in its many millions of years of existence. Our bodies are perhaps unsure of how to break it down – we’ve got enzymes and mechanisms for digesting animal fats, but some Franken-fat with a new molecular structure is a different deal entirely. What’s the result? If you’re lucky, it won’t affect you in the long run. But over time, as you eat more and more of these modified fats, your body gets worse and worse at dealing with them, and there’s fairly good evidence that trans fats contribute to certain diseases, mainly heart disease.
 Back when they figured out to hydrogenate liquid fats into solid fats, they proclaimed it as a triumph of science: no longer would we be forced to cook with lard or butter. Just slap some margarine on that bread! Make your cakes and cookies with shortening and they’ll be healthier for you! And now we’ve finally come to the end of a long road that taught us we may have been a little overzealous about proclaiming hydrogenation as a benefit to society. That’s why you hardly see it in anything anymore. Note that it naturally occurs in small amounts in animal products, and if you’re buying most any brands of grocery store peanut butter, you’ll find that one of the main ingredients is “partially hydrogenated vegetable oil” (to my ears, a death cocktail mixing the worst of two worlds, vegetable oils and trans fats, but that’s a story for another day).
Back when they figured out to hydrogenate liquid fats into solid fats, they proclaimed it as a triumph of science: no longer would we be forced to cook with lard or butter. Just slap some margarine on that bread! Make your cakes and cookies with shortening and they’ll be healthier for you! And now we’ve finally come to the end of a long road that taught us we may have been a little overzealous about proclaiming hydrogenation as a benefit to society. That’s why you hardly see it in anything anymore. Note that it naturally occurs in small amounts in animal products, and if you’re buying most any brands of grocery store peanut butter, you’ll find that one of the main ingredients is “partially hydrogenated vegetable oil” (to my ears, a death cocktail mixing the worst of two worlds, vegetable oils and trans fats, but that’s a story for another day).
Time magazine just came out with an article suggesting we have perhaps been wrong to demonize normal, saturated fats as we have over the last few decades. Over these 50 years or so, very little evidence has come to support the claim that fats are bad for you, except in this particular case of trans fats. It again brings to the mind the question of when a new technology should really be proffered by “experts” as safe. Who knows what will be next to go – unfortunately, we’ll have to wait on epidemiological data showing trends in diet and disease, and that could take decades more.
Regardless, the health craze of substituting real fats with fake fats has fallen pretty hard, and not a moment too soon. Our health as a society, nay, a species, depends on a long-overdue mass acceptance of the cold, hard, solid-at-room-temperature facts.
 When you smell something, it’s because odorant molecules are drifting through the air from the substance into your nose. If you smell pizza, it’s because molecules of pizza are flying off the pizza itself and eventually making their way into your brain where they activate certains neurons. The molecules take a pretty short trip to your brain by getting picked up by the olfactory bulb and being transmitted to the limbic system. Each of the cells that receive these smells and then transfer them are different from each other.
When you smell something, it’s because odorant molecules are drifting through the air from the substance into your nose. If you smell pizza, it’s because molecules of pizza are flying off the pizza itself and eventually making their way into your brain where they activate certains neurons. The molecules take a pretty short trip to your brain by getting picked up by the olfactory bulb and being transmitted to the limbic system. Each of the cells that receive these smells and then transfer them are different from each other.
 Whenever I smell Vienna Fingers, I think of my grandmother’s kitchen because she always had these delicious suckers hanging around, and man, did my nose enjoy them!
Whenever I smell Vienna Fingers, I think of my grandmother’s kitchen because she always had these delicious suckers hanging around, and man, did my nose enjoy them!