Epidemiology is the study of how diseases are propagated through a population. It’s funny because fear operates on a similar principle — it’s more contagious and less dangerous, but fear spreads through a population with ease. The worst part is that while contagious diseases typically come from a virus or bacterium, fear is manufactured out of thin air and spreads its fight-or-flight response unhindered and instantaneously to whomever is close enough to experience it.
We’ll talk about food in this discussion, and attempt to explain how the epidemiology of fear affects our social perceptions of what is “healthy” or “bad for you.” Even if you’re not a chemist at heart, we’ll break it down in an easily digested (see what I did there?) format. Our ultimate goal is for you to understand how and why misconceptions arise, so we won’t waste your time with esoteric chemistry lingo.
Let’s consider an ingredient in food that is widely feared, but to prevent bias we won’t give it a name just yet. Look at its chemical structure below, keeping in mind that long horizontal zig-zagging chain is just a bunch of carbon atoms linked together:
On the right side, you see “Na,” which is the chemical symbol for sodium. If you slapped a chloride onto that sodium, you’ve have table salt. Notice how it has a + sign above it, meaning that it’s slightly positively charged. Of course, a positively charged thing is attracted to a negatively charged thing. As it turns out, the sodium can bind to the oxygen (it has a minus symbol next to it), because opposites attract. But here’s an important note: this doesn’t mean these two parts are now permanently linked. The sodium and oxygen atoms here are only electrically bonded to each other, rather than a stronger type of bond (such as the ones that connect the rest of the atoms in this entire molecule. Their bond is weak and it’s broken easily.
Normally, the Na+ above is replaced with a hydrogen, and you’ll have -O H on the end there instead of -O Na. In your body, this large molecule naturally exists as the picture above. In other words, that oxygen with a negative symbol floats around in your body, and it’s able to pick up any positively charged thing it encounters. That could be hydrogen in water or, as above, a sodium atom.
You can imagine that this molecule floats around and just happens to bump into a sodium atom, at which point the two join together, at least temporarily, in electric attraction. They’ll probably separate in a fraction of a second, but they can bond for a short period.
What’s the big reveal here? Well, the molecule above is one of the twenty amino acids that make up every protein on earth. Beef, eggs, cheese, seafood, chicken, and the rest all incorporate this molecule to a huge extent. You’re getting trillions and trillions, if not more, of these molecules at every meal. In fact, for the math-oriented reader, we’ll break it down below. If you’re not a huge fan of numbers, skip this optional section.
Amount of the above amino acid in an average 3 ounce hamburger:
- The average hamburger has 24.8 grams of protein, of which 3.751 grams is our mystery molecule above. A mole of this molecule weighs 147 grams, meaning we have 0.025 moles per serving, or 2.5% of a mole.
- 2.5% of a mole is equal to one and a half septillion molecules. It’s exactly 1,536,123,809,523,809,523,809,523 molecules. The take-home message: An average hamburger has one and a half million trillion copies of this molecule. It’s unfathomably large.
So you’re getting this amino acid at every meal in unbelievably high amounts. If you’ve salted your burger, you’re also eating lots of sodium atoms, and there’s a good chance these two would come into contact naturally and bond with each other temporarily.
With that out of the way, let’s reveal our molecule: we’re talking about monosodium glutamate, or MSG. Now, before your mind jumps to anything you’ve previously been told about MSG, stick with me here and review what we’ve just discussed. Your body probably contains millions of millions of millions of monosodium glutamate right now, simply because you had protein with salt in a recent meal. So what’s the controversy?
MSG has been accused of causing many health problems, to the point where Asian restaurants proudly proclaim they don’t use it as an ingredient in their food. Whether they actually believe it’s harmful or not is irrelevant because their society believes it to be harmful. It actually stems from an anecdotal report in a 1968 issue of The New England Journal of Medicine. Here’s what the person said:
I have experienced a strange syndrome whenever I have eaten out in a Chinese restaurant, especially one that served northern Chinese food. The syndrome, which usually begins 15 to 20 minutes after I have eaten the first dish, lasts for about two hours, without hangover effect. The most prominent symptoms are numbness at the back of the neck, gradually radiating to both arms and the back, general weakness and palpitations…
Imagine reading this in a scientific journal or magazine. It might spook you and make you want to avoid whatever ingredient is causing such a reaction. You might look at Chinese food ingredients, notice that MSG is in nearly all of them, and righteously declare a war on MSG. Does this make sense? Maybe, if it’s the only common link between every dish.
What else could explain this man’s symptoms? Maybe he ate too much, or too quickly. Maybe he ate something fried in an oil that triggers an allergic reaction. Maybe he’s allergic to seafood and the pork-fried rice was contaminated. Whatever the reason, there are infinitely many “possible explanations,” and the cause warrants a bit of digging.
Consider the effects of a high-sugar meal on your body. You’ll pump out a ton of insulin to clear out your blood of excess sugar. If it clears out too much, you might end up with low blood sugar, the opposite end of the spectrum. What does low blood sugar feel like? If you’ve gone 14 or 15 hours without a meal, you probably know its symptoms: for many people, weakness or aches are both common symptoms. You might argue that this man’s reaction was not due to any particular ingredient found in Chinese food, but rather, by the massive flood of simple sugars he fed his body when he ate the rice he was served with his meal. If you’ll recall our recent discussion on the genetic variety in tolerance for rice, you’ll remember that in general, western nations don’t digest rice as easily and efficiently as those that have been eating it as a staple food at every meal for thousands of years. Perhaps there is less digestion in the mouth, which leads to large digestion in the small intestine and stomach, which could theoretically lead to a larger spike in blood glucose. From here, you might start to wonder if perhaps the rice is a viable candidate, rather than the MSG. Of course, none of what I’ve written in this paragraph has been scientifically confirmed by a double-blind, peer-reviewed study, so take it with a grain of salt; it’s just my conjecture. The point I’m trying to make here is that without scientific evidence, neither the rice nor the MSG can be conclusively linked to the anecdotal symptoms of one man in 1968.
We haven’t concluded that MSG is not harmful in the above paragraph. We’ve only concluded that it cannot be singularly pin-pointed based on current scientific consensus. In fact, that opposite is true: here are three double-blind or placebo-controlled studies debunking the harmful effects of MSG. If double-blind studies can’t determine potential harmful effects, it seems reasonable that one man writing anecdotally should not be taken as the voice of molecular reason and propagated as fact for decades.
So where does this leave us with MSG? There’s not been any evidence that it is responsible for the symptoms it’s accused of, and we have already established that this molecule is already present in your body in a number bigger than you can type into a calculator. We can’t say with accuracy how much MSG might be added to any given food dish, but even if it exceeds the amount of glutamate naturally occurring in the meal, it exists in exactly the same form in your body and will be treated exactly the same.
So what about overdosing on glutamate? Can glutamate all on its own cause these symptoms? Glutamate is itself a neurotransmitter, meaning that it’s what your brain uses to transmit signals to the rest of your body. Too much of any neurotransmitter can be damaging by what’s called excitotoxicity. The LD50 for MSG in rats is 16.6 grams per kilogram. If the average rat weighs 0.2 kilograms, it would need to eat 33 grams, or over an ounce of the stuff all at once to have a 50% chance of death. Let’s go out on a limb and say that the LD50 scales more or less with humans. In fact, let’s say that our tolerance is lower and that we can only take 12 grams of the stuff per kilogram before we die. If a 160 pound person weighs 72.5 kilograms, he or she would have to eat 870 grams of MSG all at once to die half of the time. How much is 870 grams? It’s nearing two full pounds. Look below for the final, optional math on this issue:
- If 870 grams of MSG is our theoretical LD50 for MSG in humans, and it has a density of 1.62 grams per cubic centimeter, we would have to ingest 537.03 cubic centimeters, or just over half of a cubic meter.
 Here’s what a full cubic meter looks like. If you think you can eat half of the volume of this box in MSG powder, let alone anything at all, then you’re in grave danger of MSG overdose and excitotoxicity, but you’re going to have to work against your body to force it down your throat. Of course, our LD50 for humans is one I just made up on the spot, because we know that LD50 numbers in rat shouldn’t scale directly to humans. But making MSG even more toxic to humans than rats, as we did in the above example, still illustrates the point of just how much you’d have to take to cause problems.
Here’s what a full cubic meter looks like. If you think you can eat half of the volume of this box in MSG powder, let alone anything at all, then you’re in grave danger of MSG overdose and excitotoxicity, but you’re going to have to work against your body to force it down your throat. Of course, our LD50 for humans is one I just made up on the spot, because we know that LD50 numbers in rat shouldn’t scale directly to humans. But making MSG even more toxic to humans than rats, as we did in the above example, still illustrates the point of just how much you’d have to take to cause problems.
So what’s with the title of this discussion? There’s no overt fear-mongering over this issue. Instead, it begins with an anecdote, whose message is propagated across cities, states, and eventually countries to produce a general consensus that is not based on any scientific evidence. It can happen with anything — food, rock and roll, or violent video games — but what a population believes to be true and whether it is actually true are often not the same. Always question the source, and if you’re skeptical, do just a little bit of digging. What you find may surprise you.

 They’ve been reported in both captivity and in the wild. In the wild, it’s unlikely that a polar and grizzly bear meet up because of where they live, but it could perhaps happen rarely. Look at the chart to the right and you’ll see that there many possible combinations of parent species that would produce a grolar bear. Terrifying, huh?
They’ve been reported in both captivity and in the wild. In the wild, it’s unlikely that a polar and grizzly bear meet up because of where they live, but it could perhaps happen rarely. Look at the chart to the right and you’ll see that there many possible combinations of parent species that would produce a grolar bear. Terrifying, huh?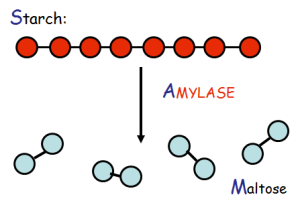




 Here we can see that ATP, which is the main energy “currency” of the body (meaning, all incoming energy sources eventually end up as ATP so they can be spent universally in the body, like having a credit card instead of Canadian dollars!) is made from many sources including gkycogen and fatty acids. Notice how quickly glycogen is turned into ATP compared to fatty acids — over five times the speed! When you’re in a fight-or-flight situation, you want the fastest possible mode of energy generation, and glycogen is the go-to guy.
Here we can see that ATP, which is the main energy “currency” of the body (meaning, all incoming energy sources eventually end up as ATP so they can be spent universally in the body, like having a credit card instead of Canadian dollars!) is made from many sources including gkycogen and fatty acids. Notice how quickly glycogen is turned into ATP compared to fatty acids — over five times the speed! When you’re in a fight-or-flight situation, you want the fastest possible mode of energy generation, and glycogen is the go-to guy.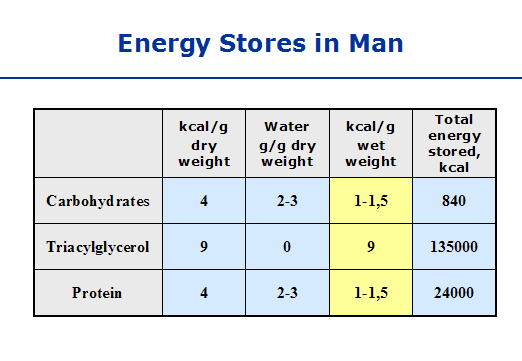 We just mentioned that you store glycogen (remember, that’s the storage form of these sugars), but you can only hold so much. Picture a big bucket with a tiny hole in the bottom — if you use your garden hose to fill the bucket half-way full, it will slowly drain out over time, and in a few hours or days the bucket will be empty. On the other hand, if you fill it up to capacity and then continue to spray water over it, the water will overflow from the top. Our glycogen stores work the same way — we can only hold so much, and we slowly burn it over the course of a day to fuel our brain and our motion. In the chart to the right we can see that we store 840 total kilocalories as glycogen at any given time. Dividing this by 4 tells us the number of grams of glycogen that is our maximum – it’s about 210 grams, and we have to add an additional 4 times this amount to account for the water weight associated with storing glycogen. This is roughly 1000 grams, or 2 pounds of quick energy!! Our glycogen bucket holds almost nothing compared to our fat stores, which, in this chart, account for about 66 pounds! Compare that to the 2 pounds for glycogen; fat is by far the most abundant energy source we have, and it’s a shame we can’t utilize it more efficiently.
We just mentioned that you store glycogen (remember, that’s the storage form of these sugars), but you can only hold so much. Picture a big bucket with a tiny hole in the bottom — if you use your garden hose to fill the bucket half-way full, it will slowly drain out over time, and in a few hours or days the bucket will be empty. On the other hand, if you fill it up to capacity and then continue to spray water over it, the water will overflow from the top. Our glycogen stores work the same way — we can only hold so much, and we slowly burn it over the course of a day to fuel our brain and our motion. In the chart to the right we can see that we store 840 total kilocalories as glycogen at any given time. Dividing this by 4 tells us the number of grams of glycogen that is our maximum – it’s about 210 grams, and we have to add an additional 4 times this amount to account for the water weight associated with storing glycogen. This is roughly 1000 grams, or 2 pounds of quick energy!! Our glycogen bucket holds almost nothing compared to our fat stores, which, in this chart, account for about 66 pounds! Compare that to the 2 pounds for glycogen; fat is by far the most abundant energy source we have, and it’s a shame we can’t utilize it more efficiently.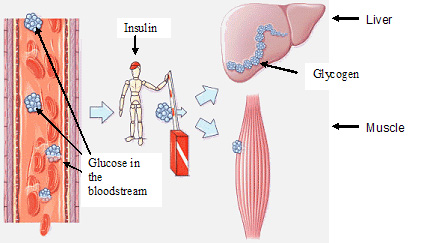
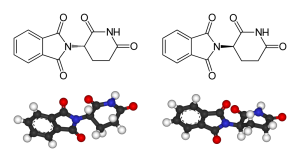 on the market decades ago, we wanted pregnant women to stop having morning sickness. Instead, the lack of research and long-term effects meant that birth defects were uncommonly high among those taking the drug. Had we taken a little more care with manufacturing or research, it may not have happened that way. The world got excited, and many newborn children paid the price of the immaturity of the compound’s data.
on the market decades ago, we wanted pregnant women to stop having morning sickness. Instead, the lack of research and long-term effects meant that birth defects were uncommonly high among those taking the drug. Had we taken a little more care with manufacturing or research, it may not have happened that way. The world got excited, and many newborn children paid the price of the immaturity of the compound’s data.
 Back when they figured out to hydrogenate liquid fats into solid fats, they proclaimed it as a triumph of science: no longer would we be forced to cook with lard or butter. Just slap some margarine on that bread! Make your cakes and cookies with shortening and they’ll be healthier for you! And now we’ve finally come to the end of a long road that taught us we may have been a little overzealous about proclaiming hydrogenation as a benefit to society. That’s why you hardly see it in anything anymore. Note that it naturally occurs in small amounts in animal products, and if you’re buying most any brands of grocery store peanut butter, you’ll find that one of the main ingredients is “partially hydrogenated vegetable oil” (to my ears, a death cocktail mixing the worst of two worlds, vegetable oils and trans fats, but that’s a story for another day).
Back when they figured out to hydrogenate liquid fats into solid fats, they proclaimed it as a triumph of science: no longer would we be forced to cook with lard or butter. Just slap some margarine on that bread! Make your cakes and cookies with shortening and they’ll be healthier for you! And now we’ve finally come to the end of a long road that taught us we may have been a little overzealous about proclaiming hydrogenation as a benefit to society. That’s why you hardly see it in anything anymore. Note that it naturally occurs in small amounts in animal products, and if you’re buying most any brands of grocery store peanut butter, you’ll find that one of the main ingredients is “partially hydrogenated vegetable oil” (to my ears, a death cocktail mixing the worst of two worlds, vegetable oils and trans fats, but that’s a story for another day). When you smell something, it’s because odorant molecules are drifting through the air from the substance into your nose. If you smell pizza, it’s because molecules of pizza are flying off the pizza itself and eventually making their way into your brain where they activate certains neurons. The molecules take a pretty short trip to your brain by getting picked up by the olfactory bulb and being transmitted to the limbic system. Each of the cells that receive these smells and then transfer them are different from each other.
When you smell something, it’s because odorant molecules are drifting through the air from the substance into your nose. If you smell pizza, it’s because molecules of pizza are flying off the pizza itself and eventually making their way into your brain where they activate certains neurons. The molecules take a pretty short trip to your brain by getting picked up by the olfactory bulb and being transmitted to the limbic system. Each of the cells that receive these smells and then transfer them are different from each other.
 Whenever I smell Vienna Fingers, I think of my grandmother’s kitchen because she always had these delicious suckers hanging around, and man, did my nose enjoy them!
Whenever I smell Vienna Fingers, I think of my grandmother’s kitchen because she always had these delicious suckers hanging around, and man, did my nose enjoy them!
 You might already know the chemical formula for table salt is NaCl. It just means for every sodium atom, there is a chloride atom to go with it. It’s a very simple substance, but it’s not a molecule — a term which is reserved for discrete groups of atoms (sugar molecules or water molecules, for example, are always the same and don’t associate with each other so much that we can’t separate them). Salt is really an ionic substance, which just means that instead of having a discrete, separate “NaCl” molecule, it builds on itself, and each sodium bonds to a couple of chlorides, and each chloride finds a couple of sodiums. The end result is a crystal, just like diamond, that’s highly ordered because all the atoms bond in the exact same way with all the others. This is why coarse salt comes in those very geometrically ordered crystals — it’s the natural way that salt comes, all sharp lines and angles.
You might already know the chemical formula for table salt is NaCl. It just means for every sodium atom, there is a chloride atom to go with it. It’s a very simple substance, but it’s not a molecule — a term which is reserved for discrete groups of atoms (sugar molecules or water molecules, for example, are always the same and don’t associate with each other so much that we can’t separate them). Salt is really an ionic substance, which just means that instead of having a discrete, separate “NaCl” molecule, it builds on itself, and each sodium bonds to a couple of chlorides, and each chloride finds a couple of sodiums. The end result is a crystal, just like diamond, that’s highly ordered because all the atoms bond in the exact same way with all the others. This is why coarse salt comes in those very geometrically ordered crystals — it’s the natural way that salt comes, all sharp lines and angles. The other key thing you might not know is that ice is not slippery. Yes, ice is not slippery, but water is. Ice on the sidewalk has a very thin layer of water on top of it, which makes it hard to walk on. Any amount of pressure or heat creates this layer, so there’s literally no way for you to walk only on ice — you’re always walking on the buffer of water above it. Now imagine dissolving some table salt into that top layer of water. Just like in a glass of water, the atoms of salt will come apart and associate with water instead. Since it’s probably below freezing at this point, the water would really, truly like to become frozen water, which some people call ice. Thanks to salt, it no longer can.
The other key thing you might not know is that ice is not slippery. Yes, ice is not slippery, but water is. Ice on the sidewalk has a very thin layer of water on top of it, which makes it hard to walk on. Any amount of pressure or heat creates this layer, so there’s literally no way for you to walk only on ice — you’re always walking on the buffer of water above it. Now imagine dissolving some table salt into that top layer of water. Just like in a glass of water, the atoms of salt will come apart and associate with water instead. Since it’s probably below freezing at this point, the water would really, truly like to become frozen water, which some people call ice. Thanks to salt, it no longer can.
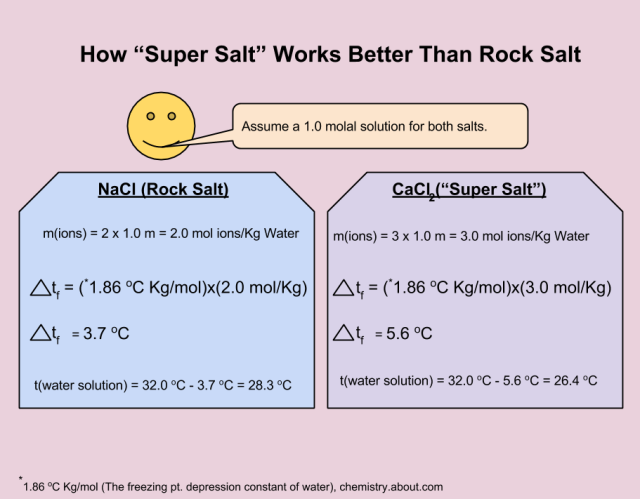



 Ever studied the brain? Your brain uses cells called neurons to send signals to your body. It works by letting in sodium ions to your brain cells, which change the electrical forces around them, resulting in impulses that travel to your muscles as movement. This seems like a good argument for eating salt, since the sodium we get from it is vital to our very existence, but there’s more to it: not only do sodium ions affect nerve impulses, but both chloride and potassium ions are vital to the mix as well. Using KCl instead of NaCl is just as beneficial for your health in terms of the essential electrolytes (anything that dissolves to make ions, in our case, table salt or KCl) your body doesn’t just make on its own.
Ever studied the brain? Your brain uses cells called neurons to send signals to your body. It works by letting in sodium ions to your brain cells, which change the electrical forces around them, resulting in impulses that travel to your muscles as movement. This seems like a good argument for eating salt, since the sodium we get from it is vital to our very existence, but there’s more to it: not only do sodium ions affect nerve impulses, but both chloride and potassium ions are vital to the mix as well. Using KCl instead of NaCl is just as beneficial for your health in terms of the essential electrolytes (anything that dissolves to make ions, in our case, table salt or KCl) your body doesn’t just make on its own. tension, the fact that sodium absorbs water means the water balance in your body is upset and your cells have lost some fluid — which is exactly why eating salt makes you really thirsty. KCl doesn’t have this problem.
tension, the fact that sodium absorbs water means the water balance in your body is upset and your cells have lost some fluid — which is exactly why eating salt makes you really thirsty. KCl doesn’t have this problem.
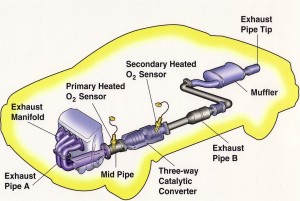

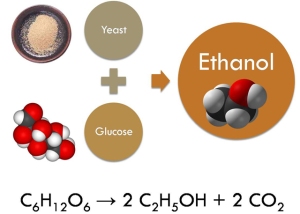
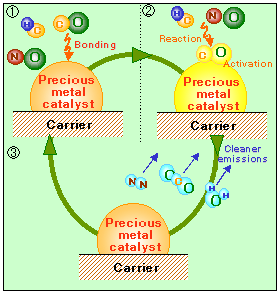 Our first reaction makes oxygen and molecular nitrogen, both of which make up the air around us, so that’s great. The second reaction makes CO2, which I suppose is good for the plants that use it for energy, but it’s not needed for us, and in fact it’s a waste product of our metabolism. The third reaction is just like setting anything on fire: you mix the fuel and some oxygen and you will always make water and carbon dioxide. So for the most part, we’re taking in unspent fuel and poisonous gases and releasing carbon dioxide, water, and oxygen, all of which are way less malicious.
Our first reaction makes oxygen and molecular nitrogen, both of which make up the air around us, so that’s great. The second reaction makes CO2, which I suppose is good for the plants that use it for energy, but it’s not needed for us, and in fact it’s a waste product of our metabolism. The third reaction is just like setting anything on fire: you mix the fuel and some oxygen and you will always make water and carbon dioxide. So for the most part, we’re taking in unspent fuel and poisonous gases and releasing carbon dioxide, water, and oxygen, all of which are way less malicious.